MEFANET Journal 2015; 3(1): 16-20
REVIEW
Synthesis of evidence of diagnostic tests and preventive programs identifying pre-diabetes type
Dagmar Tučková1,2, Miloslav Klugar1,2*, Jana Marečková1,2, Jitka Klugarová1,2
1 Department of Social Medicine and Public Health, Faculty of Medicine and Dentistry, Palacký University in Olomouc, Olomouc, Czech Republic
2 The Czech Republic (Middle European) Centre for Evidence-Based Health Care: An affiliated Centre of the Joanna Briggs Institute, Faculty of Medicine and Dentistry, Palacký University in Olomouc, Olomouc, Czech Republic
* Corresponding author: miloslav.klugar@upol.cz
Abstract
Article history:
Received 1 June 2015
Revised 28 July 2015
Accepted 10 August 2015
Available online 18 August 2015
Peer review:
Jiří Kratochvíl, Jaroslav Majerník, Jana Strenková
Download PDF
Introduction: Type 2 diabetes mellitus (T2D) has become the main type of diabetes in children and it is expected that in countries with high income diabetes it is projected to be one of the leading causes of death by 2030. Another fact is that programs and tests diagnosing pre-diabetes type 2 (T2P-DMC) are missing.
Methods: The aim of the paper is to present the steps for the synthesis of the evidence within the brand new type of the systematic review (SR): SR of diagnostic test accuracy (DTA). Using the acronym PIRD it was developed a review question, search strategy and inclusion and exclusion criteria.
Results: The initial search was done in two databases (MedLine and Cinahl) with 2 025 results. The second search after the improvement of the sensitivity and the specificity was done in 15 databases with 3 681 results.
Conclusion: This methodological paper introduces how to conduct the systematic review protocols of diagnostic test accuracy on the example of T2P-DMC.
Keywords
Type 2 pre-diabetes; children; diagnostic test accuracy
Introduction
Approximately 347 million of people around the world suffer from diabetes [1]. In 2004, an estimated 3.4 million of people died on the consequences related to fasting high blood sugar [2]. According to the research carried out in Europe [3], type 2 diabetes (T2D) prevalence in children is increasing. Although a very high prevalence of T2D has been observed in non-Caucasian groups (African Americans, Native Americans, Hispanics), T2D occurs in all races [4]. In the SEARCH study [5], the incidence rate (per 100,000 person-year) of T2D among children and adolescents varies greatly by ethnicity, with the highest rates observed among youths aged 15–19 years in minority populations. In particular, the reported incidence rate was 49.4 for Native Americans, 22.7 for Asian/Pacific Islanders, 19.4 for African Americans, 17 for Hispanics, and 5.6 for non-Hispanic whites. The increased prevalence of T2D in the obese paediatric population is paralleled by an increased prevalence of the prediabetes conditions. In particular, 25% of children and 21% adolescents with severe degree of obesity, irrespective of ethnicity, were found to have impaired glucose tolerance (IGT) [6]. The global rise of childhood obesity and physical inactivity is widely believed to play a crucial role. Healthy eating and lifestyle habits are a strong defence against the disease [7].
The effective pre-diabetes diagnostic tests, early diagnosis and preventive programs can help diabetes prevention development. The main problem in children is that there exist some recommendations regarding the diagnostic tests and preventive programs for pre-diabetes and diabetes. These have been made by the American Diabetes Association (ADA) [2], but formal screening is infrequent [3]. The diagnostic tests and preventive program options include fasting plasma glucose (FPG) and oral glucose tolerance tests (OGTTs), but both require fasting samples that the patients find inconvenient [4], and it is not clear what the best diagnostic strategy is—another reason why the screening is under-performed [5]. Childhood obesity epidemic brings a need to implement test practices for the paediatric population. The effective test practices for type 2 pre-diabetes mellitus in children (T2P-DMC) will allow us to deal with this disease in the earliest stage of the ontogenetic development and to improve public healthcare in the developed countries around the world.
The main aim of the paper is to present the design of the synthesis of the diagnostic tests for the identification of T2P-DMC followed by intervention to prevention of subsequent onset of T2D. For better evidence we decided to focus on diagnostic test accuracy (DTA) which is a new developed methodology in the field of evidence synthesis [8]. It shows the propaedeutic of such a systematic review (SR) conducting DTA which are used to identify the presence, or the absence of a condition for the purpose of developing an appropriate treatment plan [9].
Before the initial search (the part of protocol development) the preliminary search was conducted in four databases (MedLine, Prospero, JBI Library and Cochrane library) to find out if there are any existing SR of this topic. There was not find any SR or a guideline related to the issue of DTA in T2P-DMC.
Methods
The review question for our research is: Which diagnostic test is currently the most accurate in identifying T2P-DMC at the different stage of ontogenetic development?
PICO acronym was for SR of DTA replaced by more suitable acronym PIRD where P = population, problem, patient; I – index test; R – reference test; D – diagnosis of interest.
In primary studies of DTA, the test of interest (the ‘index test’) is compared to an existing diagnostic test (the ‘reference test’), which is known to be the best test currently available for identifying accurately the presence or absence of the condition of interest [8]. This PIRD acronym is according to JBI methodological approach more suitable than PICO and it was developed peculiarly for DTA methodology. It covers all parts of what we want to find in the literature. As it was said in the introduction, the diagnostic tests for detection of T2P-DMC are not standardized. That was the reason the reference tests were not described within the PIRD which was determined [8,10].
Our inclusion/exclusion criteria in PIRD acronym:
Population – children with the risk of overweight, obesity, hypokinesis and metabolic syndrome at different stages of ontogenetic development. According to International Diabetes Federation [11] (IDF, 2014), the age criteria ontogeny for metabolic syndrome development is following:
- 6–10 years
- 10–16 years
- > 16 years
Index test – studies that evaluate any type of existing pre-diabetes diagnostic practices and programs, for example impaired fasting glucose level of 100–125 mg/dL, impaired glucose tolerance: A plasma glucose level (obtained 2 hours after a 75-g oral glucose challenge) > 140 mg/dL but < 200 mg/dL or haemoglobin A1c level of 5.7–6.4%. The minimum of 3 from 5 major criteria: (obesity determined by waist circumference, hypertension, low HDL levels, elevated triglyceride levels, and glucose intolerance)
Reference test – as we mentioned in the introduction, here does not exist any reference test for children.
Diagnosis of interest – studies that include the following diagnosis of interest: type 2 pre-diabetes mellitus.
For the needs of the initial search was developed search strategy which consists of the following terms/key words. The Table 1 shows the key words and terms which were used for MedLine database. This search strategy was adapted for each database.
Table 1. Key words used for initial search in MedLine database
|
1. |
Children OR teenager* OR kids OR non adults OR early ontogenetic stages OR youngster, adolescent* OR youth |
|
2. |
Fasting glucose level OR fasting blood glucose level OR fasting plasma glucose OR FPG |
|
3. |
Impaired glucose tolerance: A plasma glucose level OR IGT |
|
4. |
H$moglobin A1c OR glycoh$moglobin A1c OR A1c h$moglobin OR Hb OR Hgb OR HbA1c OR HGBA1C |
|
5. |
Obesity OR overweight OR BMI + percentile |
|
6. |
Hypertension, high blood pressure |
|
7. |
Low HDL levels, elevated triglyceride levels, TG, triacylglycerol, TAG, or triacylglyceride |
|
8. |
Glucose intolerance |
|
9. |
Type 2 pre-diabetes mellitus OR T2P-DMC |
|
10. |
Metabolic syndrome OR metabolic syndrome X OR cardiometabolic syndrome OR syndrome X OR insulin resistance syndrome OR Reaven´s syndrome OR CHAOS |
|
11. |
Diagnosis OR detect OR accura* OR diagnostic accuracy test OR diagnostic accuracy clinical tests OR test of diagnostic accuracy OR diagnostic accuracy OR sensitivity OR specificity OR ROC |
|
12. |
Animal* |
|
13. |
1 AND (2 OR 3 OR 4) AND (5 OR 6 OR 7 OR 8) AND (9 OR 10) AND 11 NOT 12 |
The extensive systematic search strategy aims to find published and unpublished studies within the sources of both scientific literature and so called ‘grey literature’, government reports, dissertations, editorial etc. The search strategy used in this SR will include three steps according to JBI methodology [12]. Initial search has been done in MedLine and Cinahl database. This phase will be followed by two other steps. The next one will be to create the search strategy for each specific database included in the protocol. Each database has its own specific vocabulary/dictionary (engine). The third step will involve the review of the reference list of all studies that are retrieved for appraisal to search for additional studies.
Studies, which will be found, will be assessed by two independent reviewers in terms of relevance according to specified criteria. Then the standardized critical appraisal tools of JBI-DATARI will be used for critical appraisal done again by two independent reviewers. For the reviews of DTA we will use critical appraisal sheet using QUADAS-2 signalling questions [8].
Table 2. QUADAS-2 signalling questions [8]
|
Critical appraisal questions |
|
Domain 1: Patient selection |
|
- Was a consecutive or random sample of patients enrolled? |
|
- Was a case-controldesign avoided? |
|
- Did the study avoid inappropriate exclusions? |
|
Domain 2: Index test |
|
- Were the index test results interpreted without knowledge of the results of the reference standard? |
|
- If a threshold was used, was it pre-specified? |
|
Domain 3: Reference test |
|
- Is the reference standard likely to correctly classify the target condition? |
|
- Were the reference standard results interpreted without knowledge of the results of the index test? |
|
Flow and timing |
|
- Was there an appropriate interval between the index test and reference standard? |
|
- Did all patients receive the same reference standard? |
|
- Were all patients included in the analysis? |
The critical appraisal questions will be answered as “yes”, “no”, or “unclear”. When this will be not applicable “not applicable”. How much weight is placed on specific critical appraisal questions will vary between reviews and it is up to the reviewers to set what criteria, if any, will result in the exclusion of a study from the review. Many reviewers specify a set of questions which must be answered “yes” or the study will be excluded. It is important that these criteria will be applied consistently across studies [8]. Any disagreements that arise between the reviewers both during paper retrieval and during critical appraisal will be resolved through a discussion, or with a third reviewer.
Next step will be to make data extraction. The decision threshold that was used to classify the results as positive or negative is an item of data unique to studies of DTA. For data extraction will be used a JBI tool called DATARI which is based on the standards for the reporting of diagnostic accuracy studies (STARD) checklist. All studies of DTA that comply with the STARD statement will include a 2×2 table with sensitivity, specificity, and predictive values that classify patient test results and disease status [8].
Data synthesis will be done after data extraction. The results of SR of DTA can be graphically represented through two different major ways. The first way is the use of forest plots. However, in order to present data on DTA “paired” forest plots must be used where two forest plots are presented side by side; one for sensitivity and the other for specificity. In this way these graphs display the means and confidence intervals for sensitivity/specificity for each of the selected primary studies [8].
Results and discussion
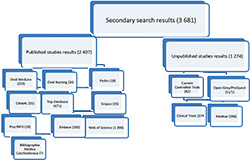
Results should bring the synthesis of findings of DTA of existing practices identifying T2P-DMC. So far the review question was developed by PIRD acronym. Specific criteria for inclusion and exclusion of studies were determined also by using of PIRD acronym. Key words for the search strategy were found and set into the searching strategy process. After we found out that there is not any existing SR for this topic (in preliminary search) we made initial an systematic literature search which was performed in MedLine and Cinahl databases. This search was done to verify whether the determined search strategy using determined key words and terms have balanced sensitivity and specificity. After this search, we got the initial search result of 2 025 studies – 958 from MedLine, and 1 067 from Cihnal. This was followed by secondary search. For this secondary search specificity was improved in search strategy and adjusted for every particular database. The second step of the search identified 3 681 studies in 15 databases. 2 407 published results and 1 274 unpublished results were found. As it is shown in the Figure 1, most published results were found in WoS (1 390), Trip Databas (471), Ovid MedLine (223), and Embase (182). Other results were found in Cinahl (55), Scopus (35), Ovid Nursing (24), Perdo (19), PsycINFO (10), and Bibliographica Medica Czechoslovaka (7). In unpublished databases we found 596 in MedNar, 574 in Clinical Trials, 82 in Current Controlled Trials, 11 in Open Grey, 1 in ProQuest. In COS Conference Papers was not found any result.
These are just the partial results. Another step will follow those in the process of SR development. This phase will be followed by selection of relevant studies, which will have two phases, a title and abstract screening and full texts screening. Then critical appraisal of relevant studies will be done, followed by data extraction of high quality studies and data synthesis.
Conclusion
The use of newly developed JBI methodology of DTA will help to analyse the situation in research field of interest. This approach will pool and synthesize relevant data which will be used for practice, healthcare policy and other stakeholder’s information.
SR provides the highest level of scientific evidence. The issue of T2P-DM is currently one of the most discussed topics because of children´s life style. And if there exist DTA helping to detect T2P-DM in adults this could work for children as well with the respect of their ontological development and metabolic rates. With early identification of T2P-DM using diagnostic tests it will be possible to prevent T2P-DM in children and teenagers. This could represent one of the solutions of this medical issue and become prevention for this disease. The consequences can further have the positive effect and the positive results could be expected in a long-term run in many fields of health and healthcare.
This paper was focused on presentation of brand new methodology and it shows the process of DTA SR development. It can be used as an initial inspiration for students of medical faculties doing their own research in the field of SR. As well as it can be pointed out that ability to develop the SR should be part of medical professional´s skills. There should be a consideration to integrate subject aiming to SR development training into a curricula at the medical faculties.
Acknowledgements
The project was supported by the research grant IGA_LF_2015_024 (Faculty of Medicine and Dentistry, Palacký University in Olomouc).
References
1. Danaei G, Finucane MM, Lu Y, Singh, MM, Cowan MJ, Paciorek CJ, Stevens GA. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378 (9785): 31-40. ISSN 0140-6736
2. WHO Global health risks: mortality and burden of disease attributable to selected major risks (1st ed.). World Health Organization: Geneva 2010.
3. Haines L, Wan KC, Lynn R, Barrett TG, Schield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care 2007; 30(5): 1097-1101.
4. Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. Type 2 diabetes in children and adolescents. Pediatr Diabetes 2009; 10(12): 17-32.
5. Writing Group for the SEARCH for Diabetes in Youth Study Group, Dabelea D, Bell RA, D'Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA 2007; 297(24): 2716-2724.
6. Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. New Engl J Med 2002; 346(22): 1756-1756.
7. World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization: Geneva 2010.
8. Campbell J, Klugar M, Ding S, Carmody DP, Hakonsen, SJ, Jadotte YT, Munn Z. Diagnostic test accuracy systematic review: The Joanna Briggs Institut´s approach. International Journal of Evidence-Based Healthcare. In press.
9. White S, Schultz T, Enuameh Y. Synthesizing evidence of diagnostic accuracy. Lippincott Williams and Williams: Philadelphia 2011. ISBN 978-1-451163-88-9.
10. Campbell J, Klugar M, Ding S, Carmody D, Hakonsen S, Jadotte Y, et al. The Systematic Review of studies of diagnostic test accuracy. In: JBI, editor. Joanna Briggs Institute Reviewers’ Manual: 2014 edition/supplement. The University of Adelaide: Adelaide 2015. In press.
11. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013; 36(Suppl. 1): S67-S74.
12. The JBI. Joanna Briggs Institute Reviewer´s Manual: 2014 edition. University of Adelaide: The JBI 2014. ISBN 978-1-920684-11-2
Please cite as:
Tučková D, Klugar M, Marečková J, Klugarová J. Synthesis of evidence of diagnostic tests and preventive programs identifying pre-diabetes type. MEFANET Journal 2015; 3(1): 16-20. Available at WWW: http://mj.mefanet.cz/mj-20150601.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License (http://creativecommons.org/licenses/by-nc-sa/3.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the MEFANET Journal, is properly cited. The complete bibliographic information, a link to the original publication on http://www.mj.mefanet.cz/, as well as this copyright and license information must be included.